Pneumococcus Antisera
Intended for in vitro diagnostic testing in clinical microbiological laboratories, reference laboratories, and the pharmaceutical industry for the quality control of vaccines.

SSI Diagnostica offers a wide range of pneumococcus antisera.
Our high-quality antisera have been a decisive factor in vaccine development over many decades. We offer high titer antisera for the effective and reliable QC testing of vaccines.
All SSI Diagnostica latex products are called ImmuLexTM (a contraction of Immunology and latex). The polyclonal pool, type and factor antisera are purified and coupled to blue latex particles. All of the products are absorbed free of cross-reactions.
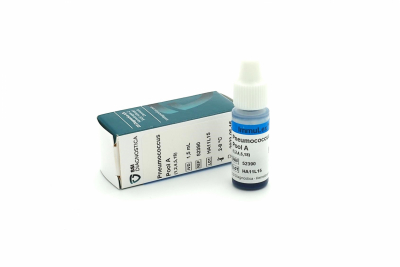
Each product is supplied in a plastic bottle with 1.5 ml ready-to-use latex reagent, sufficient for approx. 75 tests.
Pool antisera are a mixture of antisera. Pools A-I contains antisera against all serotypes, while pools P-T are mixtures of antisera against the 23-valent vaccine serotypes. By combining the two pool systems (ImmuLexTM Pneumotest-Latex), the 23 serotypes can be serotyped easily to type/group level.
Pneumococcus Antisera Solutions

High Titer Pneumococcal Antisera
High Level & Long Lasting Affinity with High Titer Pneumococcal Antisera
Read more
Clinical relevance
Serotyping of pneumococci is used for surveillance of serotype distribution in connection with pneumococcal vaccination of both children and adults. Following the introduction of the various vaccines, an increased incidence of serotypes which were not previously so dominant has been observed.
Background
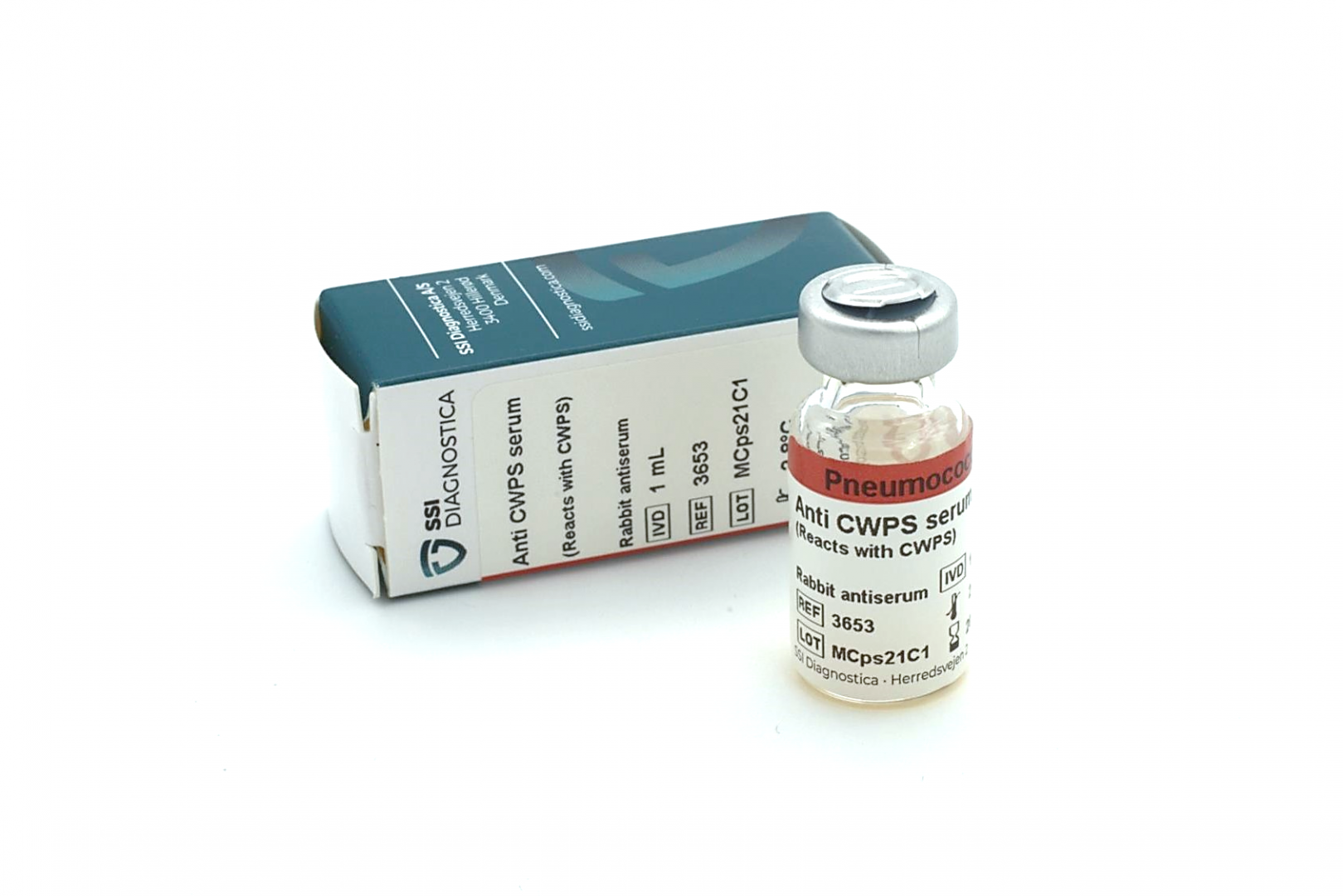
Pneumococcal antisera from SSI Diagnostica are intended for in vitro diagnostic testing in clinical microbiological laboratories, in reference laboratories, and in the pharmaceutical industry for the quality control of vaccines. All antisera are polyclonal and produced by immunising rabbits with well-defined reference strains. The specific pool, group and type antisera are absorbed free of cross-reactions. Factor antisera are absorbed free of cross-reactions within the group.
Competence
Thanks to our many years of experience producing and serotyping pneumococci, we are able to offer reliable advice and serotyping.
Quality
SSI Diagnostica’s antisera are high quality, CE-marked and produced in accordance with DS/EN ISO 13485. The shelf life is 4 years from the date of production, and a minimum of 2 years from delivery.
Simpler for the better
If you work within the field of microbiology, you might be familiar with Occam’s Razor. At SSI Diagnostica, we champion a slightly adapted version of this principle:
The simplest solution is usually the best one.
Other interesting content
Looking for something else? You might be interested in this as well.
Contact
Please, reach out if you have questions, comments or requests.